- Terms of Use
- Privacy Policy
- Cookie Policy
- © 2024 ASPA, Inc. All Rights Reserved
- Terms of Use
- Privacy Policy
- Cookie Policy
- © 2024 ASPA, Inc. All Rights Reserved
October 2024

Dear Canavan Community,
Aspa Therapeutics, a BridgeBio company, provided an update about the CANaspire investigational gene therapy clinical trial for Canavan disease during a presentation at the 31st Annual Congress of the European Society of Gene and Cell Therapy (ESGCT) in Rome, Italy. Results from the first 11 of the 12 participants who received the investigational therapy, BBP-812, were presented in an invited, peer-reviewed talk by the trial’s lead investigator, Dr. Florian Eichler, M.D., Director of the Leukodystrophy Service at Massachusetts General Hospital, Center for Rare Neurological Disease.
Key Findings:
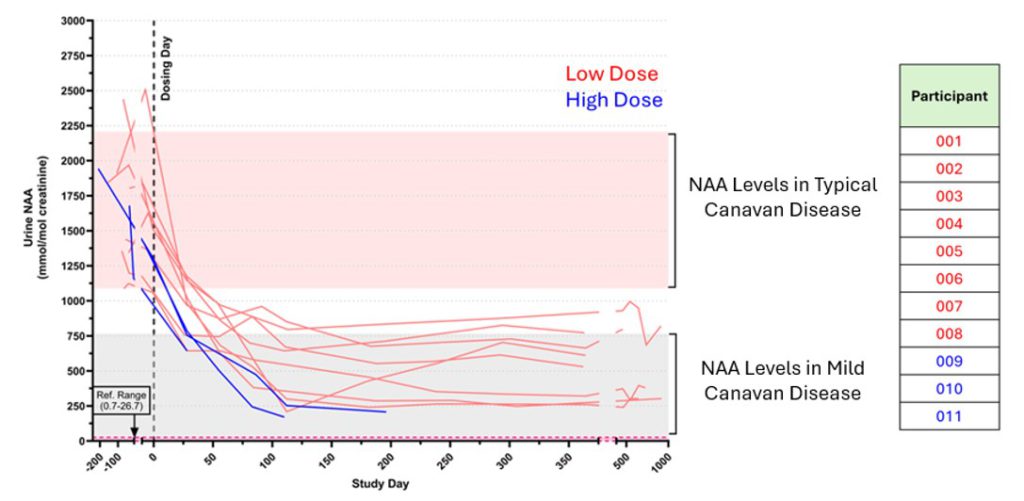
N-acetylaspartate (NAA)
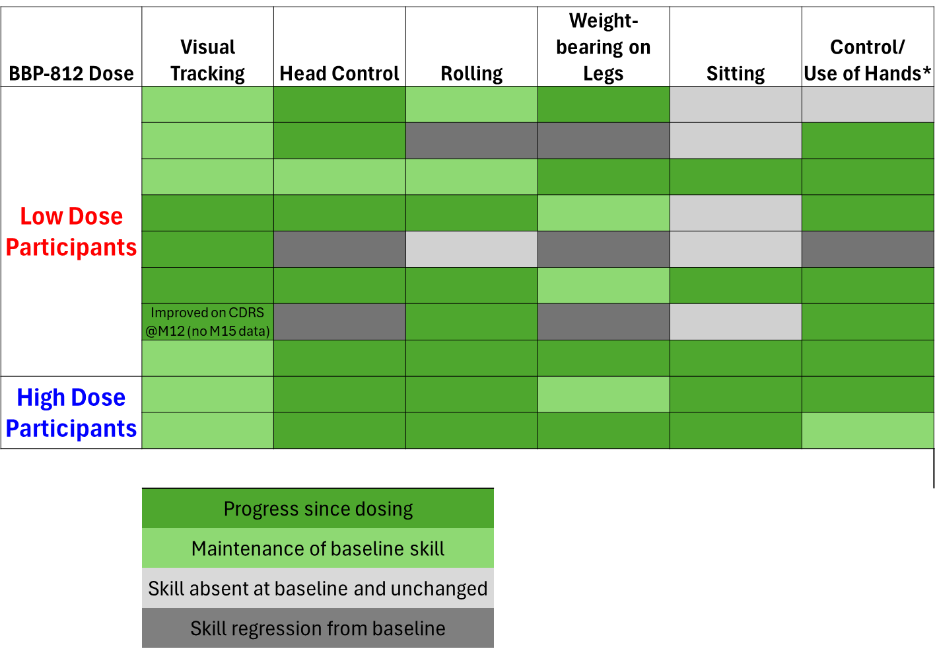
Motor Function
Magnetic Resonance Imaging (MRI): White Matter
Safety
Additional Information
Aspa Therapeutics continues to enroll new participants for the CANaspire gene therapy trial. To be considered for screening for potential participation, a child must meet the following criteria:
While the data reported here are still early and the final safety and efficacy profile of the investigational gene therapy remains to be fully established, BridgeBio believes these data show the potential of BBP-812. If you have any questions or comments, please reach out to us at canaspire@aspatx.com.
We are grateful for the continued partnership with advocacy organizations and families of the Canavan community as we work together to advance a meaningful therapeutic option for affected children. Special thanks to all the children and families who have participated or expressed interest in participating in this important research.
Sincerely,
The Aspa Therapeutics Team
You are leaving our website and we cannot be held responsible for the content of external websites.