

Gene therapy is an approach for treating genetic diseases. Gene therapies are designed to provide a working copy of a gene to perform the function of a faulty gene in the body.
In Canavan disease, the ASPA gene is not functioning correctly. The ASPA gene provides the instructions for making aspartoacylase, an essential protein that breaks down N-acetyl-L-aspartic acid (NAA). If NAA is not broken down it builds up in a child’s brain and may prevent the proper formation of myelin. Myelin insulates and protects nerve cells in the brain, and without it nerve cells are unable to send messages properly, causing difficulty with movement, language, and vision. Gene therapy for Canavan disease aims to provide a functional copy of the defective ASPA gene. The CANaspire clinical trial is designed to test the safety of Aspa’s investigational gene therapy and to determine if it might potentially benefit Canavan patients by addressing the underlying genetic cause of the disease.
Gene therapy is being extensively studied around the world, with approximately 3,500 patients having received AAV gene therapy in research and commercial settings. Gene therapies have been approved by regulators in Europe and the U.S., including two approved AAV gene therapies in the U.S., one of which is for a rare neurological condition in children. It is important to consider safety of any investigational therapy, and families should thoroughly discuss potential risks and benefits of any clinical trial with the study doctors. The American Society of Gene and Cell Therapy has additional gene therapy information here.
For CANaspire specifically, Aspa’s investigational gene therapy has been studied for safety and effectiveness in animal models but has not yet been studied in humans. The CANaspire clinical trial is designed to study safety and potential effectiveness of this investigational gene therapy in Canavan disease patients.
Aspa’s investigational gene therapy uses an AAV9 vector to deliver a functional copy of the ASPA gene. Dr. Guangping Gao, a pioneer in Canavan disease and AAV9 gene therapy, designed Aspa’s investigational gene therapy and showed that it improves the signs of Canavan disease and extends survival in animal models of the disease. These results have been presented at scientific meetings and published in a number of peer-reviewed journals. The safety and potential benefits of Aspa’s investigational gene therapy in Canavan patients are not yet known and will be studied in CANaspire.
The AAV9 vector Aspa is using for its investigational gene therapy has been studied in other rare disease gene therapy clinical trials, and is the vector used in an approved gene therapy product for a rare neurological disease in children. Evidence from animal and human studies shows that the AAV9 vector when given intravenously (IV) gets into the brain and spinal cord as well as tissues throughout the body. It is important to note, however, that use of the AAV9 vector is only one factor among many that can impact the safety and effectiveness of a gene therapy. Earlier success in other diseases does not mean that an AAV9 investigational gene therapy for Canavan disease will be safe or effective.
CANaspire is designed to investigate treatment of children with Canavan disease who are 30 months of age or younger because treating early may have the greatest impact on the course of the disease. The initial focus on younger patients is based on animal studies in a Canavan disease model, consultation with Canavan disease experts and guidance from regulatory agencies. This is similar to the approach used for initial clinical trials in other rare childhood diseases, and the results may help pave the way for studies with older patients at some point in the future.
We recognize that parents and caregivers of older children are also very interested in this investigational treatment. Once we are able to review data from our initial patients, we will work together with regulators and study doctors to assess potential opportunities for older Canavan patients to receive the Aspa investigational gene therapy as well.
Before a child can be screened for CANaspire, the child’s parents or legal guardians will have details of the study (including risks and potential benefits) explained to them in a process called informed consent. Once the parents or guardians understand what is involved and have provided written consent, the child becomes enrolled as a CANaspire participant and can begin the screening process. During screening, the child undergoes preliminary tests and is evaluated by a study doctor and study staff to see if the child is eligible to receive the investigational gene therapy.
Aspa and the study doctors, working closely with the Canavan advocacy community, have selected measures for identifying treatment-related changes that are meaningful for Canavan patients and their families and caregivers as well as clinicians. Standardized tests that measure motor, cognitive, and language development will be performed, along with laboratory tests and brain scans that can measure changes associated with restored ASPA gene function. Other tests specifically designed for Canavan disease and quality of life assessments for families and caregivers will also be used. This information may then be compared with data collected from CANinform to measure the effects of treatment on the course of the disease over time.
No, there will not be any cost to families who participate in CANaspire. Travel, lodging, and other study-related expenses will be paid for by Aspa. There will be no charge for the site visits that are associated with the trial.
The CANaspire study does not require children receiving the investigational gene therapy to have any changes to other medications or supplements.
Gene therapies are commonly given IV. This involves delivering the treatment into a blood vessel just below the skin instead of through the spine or skull. Animal research performed by Aspa has shown that giving the AAV9 gene therapy through an IV offers more efficient delivery and expression of the gene therapy in the brain and throughout the body. The company presented findings that support this approach in 2019 at the European Society for Gene and Cell Therapy Annual Congress.
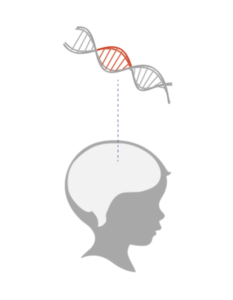
NAA is found mostly in neurons. If NAA is not broken down it accumulates in a child’s brain and may prevent the proper formation of myelin, the protective covering that helps neurons communicate with each other.
The deficits in myelin are thought to cause Canaan’s effects on movement, lanquage and vision.
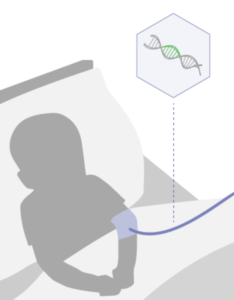
Aspa’s investigational gene therapy is given in a single intravenous dose and aims to provide working copies of the
ASPA gene throughout a child’s body.
The treatment uses an AAV9 viral vector designed to deliver the functional ASPA gene throughout the body and the brain.
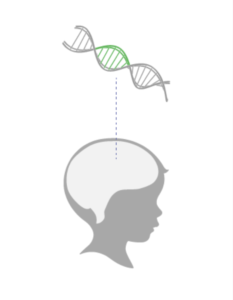
Once the functional ASPA gene is expressed, it may help restore myelin in the brain. This can potentially improve the course of Canavan disease by addressing the underlying genetic cause.
For more information about CANaspire please visit www.clinicaltrials.gov
You are leaving our website and we cannot be held responsible for the content of external websites.