

A natural history study is a non-interventional (observational) study where doctors follow patients to see how a disease progresses over time. This may also include review of past medical records. These studies are often conducted in rare diseases where there are limited data available on how a disease progresses.
The study will provide information that will greatly increase understanding of Canavan disease. Once the study is complete, Aspa will make the data publicly available to doctors, researchers and the Canavan community to share knowledge about the disease that may help advance the development of new treatments.
Any child with Canavan disease is welcome to participate in this study. For families who have lost a child to Canavan disease, Aspa has established a process whereby the family can still participate in CANinform, as data in their child’s medical record can be very informative and could contribute to progress in overall Canavan research.
All participants in CANinform will be assigned to a clinical site. Prior to enrollment, families will be asked to review and sign an appropriate consent form. The family will be asked to provide all available medical records to the clinical site in advance, to allow the study doctor to become familiar with the patient’s medical history.
For patients living in the US and certain other countries, Aspa is offering a free medical record retrieval service to help families obtain their child’s records.
Please see FAQ “What will happen during a CANinform study visit?”
Not if you live in the United States or some other countries, outside of the EU. Aspa has hired a company that can obtain the medical records on your behalf. Families living in the US or outside of the EU can call 1-833-764-2267 or 1-617-861-4617 or email CanavanMedRec@veristat.com and provide consent, and the company will handle all the details and provide you with all the necessary records necessary for participation in CANinform. This service is free of charge to families.
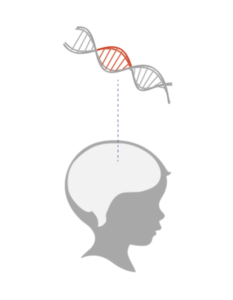
NAA is found mostly in neurons. If NAA is not broken down it accumulates in a child’s brain and may prevent the proper formation of myelin, the protective covering that helps neurons communicate with each other.
The deficits in myelin are thought to cause Canaan’s effects on movement, lanquage and vision.
Aspa’s investigational gene therapy is given in a single intravenous dose and aims to provide working copies of the
ASPA gene throughout a child’s body.
The treatment uses an AAV9 viral vector designed to deliver the functional ASPA gene throughout the body and the brain.
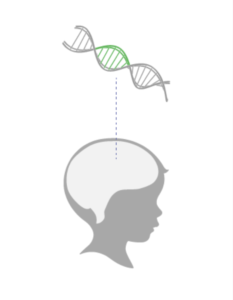
Once the functional ASPA gene is expressed, it may help restore myelin in the brain. This can potentially improve the course of Canavan disease by addressing the underlying genetic cause.
For more information about CANaspire please visit www.clinicaltrials.gov
You are leaving our website and we cannot be held responsible for the content of external websites.